HIU-Newsletter
You a scientist yourself? A journalist, a political decision-maker or business representative? In our newsletters we compile the latest battery research news for you. Specially tailored to your personal area of interest.
The research group „Electrochemistry of Materials and Interfaces” addresses challenges related to materials for energy storage devices with particular focus on the phenomena occurring at interfaces, in order to gain fundamental understanding that can be exploited to in practical systems.
Rechargeable Li-ion batteries have revolutionized the consumer electronic market and are currently powering the transition to electromobility. However, the limitations imposed by the current materials – in terms of performance, safety and sustainability – drive the need for new electrodes as well as electrolytes. The interface between these two basic battery components becomes also critical to profit from the great potential of novel battery chemistries, which are becoming more and more relevant not only for electric transportation but also for stationary energy storage. The group is extensively involved in the development of strategies to tailor the properties of battery interfaces (and interphases), from the synthesis of artificial electrode coatings to the implementation of solid and highly concentrated electrolytes. The goal is to understand how the interplay between chemical/electrochemical reactions, structural/compositional characteristics and conduction/ageing mechanisms affect the electrochemical performance of the battery.

In addition to its own equipment, the “Electrochemistry of Materials and Interfaces” research group also has access to a wide range of different analytical measurement methods and techniques at KIT and Ulm University.
(information will be uploaded shortly)
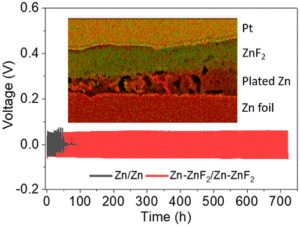
Compared to conventional lithium-ion batteries, solid electrolyte batteries (SSB) increase both the energy parameters and the safety. The main technical obstacle to the market maturity of SSB is the phase boundary solid (electrodes)/solid (electrolyte). To improve this, i.e. to homogenise and reduce the resistance, a hybrid electrolyte system for anode and cathode is being developed based on the solid electrolyte (SE) and Ionic Liquids (IL). The IL part, due to its liquid gel-like aggregate state, takes over the complete connection of the FE to the electrodes.
The main economic obstacle is the cost of the Li film. In this project, the extremely expensive metallic Li anode foil is not used and the Li anode is formed in-situ during the formation process. A bipolar battery design with a thin bipolar foil to be developed in the combination +Al/Cu(Ni)- further increases the energy parameters. The proposed solutions are particularly suitable for traction batteries due to the high energy and safety of the battery.
All-solid-state batteries (ASSBs) are promising candidates as energy storage device for many different applications, especially if safety and robustness are of importance. However, the development of ASSBs is still at an early stage. A major hindrance is the poor understanding of the processes occurring at the electrode|electrolyte interfaces, which largely originates from the lack of appropriate cell setups for their investigation. For this purpose, the use of a suitable reference electrode (RE) is crucial, i.e., an RE which is characterized by a well-defined potential that does not shift with time. However, commercially available lab test cells do not offer the option to embed such an RE. Furthermore, there is a lack of knowledge regarding the optimal RE chemistry for the different solid electrolytes. This non-ideal situation is addressed by the project partners by aiming for the following goals: (i) the development of a space-saving, experimental setup for contacting the test cell and adjusting the sample temperature and the stack pressure; (ii) the development of air tight test cells, enabling the use of an RE in a reproducible, user-friendly way, which can also be used for measurements without an RE; (iii) the optimization of the RE chemistry and geometry as well as the relative electrode configuration inside the test cell for the most important solid electrolyte classes (e.g. thiophosphates and polymers); and (iv) the elaboration of test procedures for obtaining artefact-free measuring results. The achievement of the goals (iii) and (iv) enables the user to work with a non-shifting, stable RE of the second kind with a well-defined, reproducible electrode potential instead of being forced to use a so-called pseudo-RE. The focus of this project lies on lithium-based ASSBs. Nonetheless, the possibility to transfer the results obtained and knowledge gained to other battery technologies will serve as additional guideline for the design of such cells.
The aim of the project is to develop innovative supercapacitors or ultracapacitors (EDLCs) that enable high operating voltages of up to 3.4 V at simultaneously high temperatures (>60 °C). The focus here will be on the actual behavior of novel materials in industrial cell formats. Of particular interest is the gas evolution at temperatures above 50 °C and voltages above 3 V, as this could hardly be predicted in laboratory cells so far but plays a major role in industrial cell formats.
The project thus aims to increase the power and energy density of ultracapacitors by up to 42% while reducing costs in the application (through reduced cooling system requirements).
To achieve this goal, the promising materials identified in the ULTIMATE project (grant number 03ET6131) (including curved graphene with 60% increased energy density) should be digitally simulated using advanced methods, and electrochemically and physically analyzed to identify the mechanisms of gas evolution between electrode and electrolyte. Subsequently, based on the results, new material combinations – especially in the area of binder and electrolyte – should be tested on a laboratory scale and demonstrated in industrial cell formats.
As sub-project, HIU should develop novel binder combinations based on natural polymers and suitable cross-linkers to increase the areal electrode loading while ensuring mechanical and chemical stability at high voltages and high temperatures.
The group has an established collaboration network with both German and international companies, in particular with the automotive industry. In the past years multiple collaboration projects have been carried out in cooperation with Samsung, Hyundai, BMW and Daimler.



In addition to its own equipment, the “Electrochemistry of Materials and Interfaces” research group also has access to a wide range of different analytical measurement methods and techniques at KIT and Ulm University.
Compared to conventional lithium-ion batteries, solid electrolyte batteries (SSB) increase both the energy parameters and the safety. The main technical obstacle to the market maturity of SSB is the phase boundary solid (electrodes)/solid (electrolyte). To improve this, i.e. to homogenise and reduce the resistance, a hybrid electrolyte system for anode and cathode is being developed based on the solid electrolyte (SE) and Ionic Liquids (IL). The IL part, due to its liquid gel-like aggregate state, takes over the complete connection of the FE to the electrodes.
The main economic obstacle is the cost of the Li film. In this project, the extremely expensive metallic Li anode foil is not used and the Li anode is formed in-situ during the formation process. A bipolar battery design with a thin bipolar foil to be developed in the combination +Al/Cu(Ni)- further increases the energy parameters. The proposed solutions are particularly suitable for traction batteries due to the high energy and safety of the battery.
All-solid-state batteries (ASSBs) are promising candidates as energy storage device for many different applications, especially if safety and robustness are of importance. However, the development of ASSBs is still at an early stage. A major hindrance is the poor understanding of the processes occurring at the electrode|electrolyte interfaces, which largely originates from the lack of appropriate cell setups for their investigation. For this purpose, the use of a suitable reference electrode (RE) is crucial, i.e., an RE which is characterized by a well-defined potential that does not shift with time. However, commercially available lab test cells do not offer the option to embed such an RE. Furthermore, there is a lack of knowledge regarding the optimal RE chemistry for the different solid electrolytes. This non-ideal situation is addressed by the project partners by aiming for the following goals: (i) the development of a space-saving, experimental setup for contacting the test cell and adjusting the sample temperature and the stack pressure; (ii) the development of air tight test cells, enabling the use of an RE in a reproducible, user-friendly way, which can also be used for measurements without an RE; (iii) the optimization of the RE chemistry and geometry as well as the relative electrode configuration inside the test cell for the most important solid electrolyte classes (e.g. thiophosphates and polymers); and (iv) the elaboration of test procedures for obtaining artefact-free measuring results. The achievement of the goals (iii) and (iv) enables the user to work with a non-shifting, stable RE of the second kind with a well-defined, reproducible electrode potential instead of being forced to use a so-called pseudo-RE. The focus of this project lies on lithium-based ASSBs. Nonetheless, the possibility to transfer the results obtained and knowledge gained to other battery technologies will serve as additional guideline for the design of such cells.
The aim of the project is to develop innovative supercapacitors or ultracapacitors (EDLCs) that enable high operating voltages of up to 3.4 V at simultaneously high temperatures (>60 °C). The focus here will be on the actual behavior of novel materials in industrial cell formats. Of particular interest is the gas evolution at temperatures above 50 °C and voltages above 3 V, as this could hardly be predicted in laboratory cells so far but plays a major role in industrial cell formats.
The project thus aims to increase the power and energy density of ultracapacitors by up to 42% while reducing costs in the application (through reduced cooling system requirements).
To achieve this goal, the promising materials identified in the ULTIMATE project (grant number 03ET6131) (including curved graphene with 60% increased energy density) should be digitally simulated using advanced methods, and electrochemically and physically analyzed to identify the mechanisms of gas evolution between electrode and electrolyte. Subsequently, based on the results, new material combinations – especially in the area of binder and electrolyte – should be tested on a laboratory scale and demonstrated in industrial cell formats.
As sub-project, HIU should develop novel binder combinations based on natural polymers and suitable cross-linkers to increase the areal electrode loading while ensuring mechanical and chemical stability at high voltages and high temperatures.
The group has an established collaboration network with both German and international companies, in particular with the automotive industry. In the past years multiple collaboration projects have been carried out in cooperation with Samsung, Hyundai, BMW and Daimler.
 Dr. Alberto Varzi Electrochemistry of Materials and Interfaces Tel: +49 (0731) 50 34107 Mail: alberto.varzi(at)kit.edu
Dr. Alberto Varzi Electrochemistry of Materials and Interfaces Tel: +49 (0731) 50 34107 Mail: alberto.varzi(at)kit.edu You a scientist yourself? A journalist, a political decision-maker or business representative? In our newsletters we compile the latest battery research news for you. Specially tailored to your personal area of interest.




Helmholtz Institute Ulm Electrochemical energy storage (HIU)
Helmholtzstraße 11
89081 Ulm
Germany
Tel.: +49 0731 5034001
Fax: +49 (0731) 50 34009